43 which identifier is used to mark the label of a controlled substance
MedCal CH13: Reading Medication Labels Flashcards | Quizlet Controlled substance schedule: None stated MethylPREDNISolone 40 mg per mL (when mixed) powder (must be diluted before use) Reconstitute with 1.2 mL of Bacteriostatic water for injection with benzyl alcohol ... Should a slash mark be used to denote per. tablets, capsules, liquids, suppositories, ointments, solutions, powder, granular form, or ... Statutes & Constitution :View Statutes : Online Sunshine (1) A law enforcement agency shall prepare a report identifying each prescribed controlled substance listed in Schedule II, Schedule III, or Schedule IV of s. 893.03 which is found on or near the deceased or among the deceased’s possessions. The report must identify the person who prescribed the controlled substance, if known or ascertainable.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (a) Only persons who are registered with DEA under section 303 of the Act (21 U.S.C. 823) to handle Schedule I or II controlled substances, and persons who are registered with DEA under section...
Which identifier is used to mark the label of a controlled substance
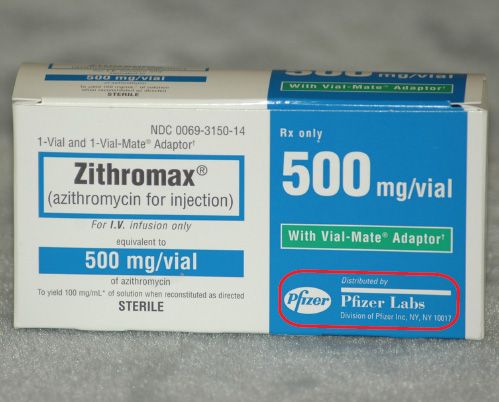
CFR - Code of Federal Regulations Title 21 - Food and Drug ... Mar 29, 2022 · (i) For each controlled substance in bulk form to be used in (or capable of use in) the manufacture of the same or other controlled or non-controlled substances in finished form, the inventory shall include: (A) The name of the substance and (B) The total quantity of the substance to the nearest metric unit weight consistent with unit size. PDF Controlled Substance FAQ - CVMA InLine Controlled substances are clearly marked with a "C" on the vial, syringe, or container that they are packaged in by the manufacturer. If you are unsure as to whether or not a substance in controlled, you can also check the Federal Controlled Substances Act (CSA) drug schedules. Medical Assisting Pharmacology Test Flashcards | Quizlet Which identifier is used to mark the label of a controlled substance? "C" followed by a Roman numeral How often should the inventory of controlled substances take place at a medical facility? Once a day What is the best way to dispose of an expired controlled substance? By using a DEA-approved disposal company What does it mean to dispense a drug?
Which identifier is used to mark the label of a controlled substance. Controlled Substances - Chemistry - CrimeLab - Alaska No controlled substance is identified in the first item. ... The statement only relates to the weight of the substance, not its identification. ... laboratory if any submitted items have been recovered from a body cavity and mark the evidence with a biohazard warning label or symbol. Gloves should be used when handling drug evidence. DCMI: DCMI Metadata Terms - Dublin Core Jan 20, 2020 · Label: The human-readable label assigned to the term. URI: The Uniform Resource Identifier used to uniquely identify a term. Definition: A statement that represents the concept and essential nature of the term. Type of Term: The type of term: property, class, datatype, or vocabulary encoding scheme. Use of Symbols in Labeling | FDA The final rule permits the use of symbols in all medical device labeling without adjacent explanatory text (referred to as "stand-alone symbols") if certain requirements are met. The final rule ... PDF Appendix A Schedules of Drugs Top 200 Brand Name drugs List ... - Virginia 1 Identify the facilities that may use unit dose carts 2 Understand the use and functions of unit dose carts B Repackaging ... Information on Prescription Stock Bottle Labels Controlled substance mark Lot number Expiration date National Drug Code (NDC) number Prescription-only symbol (Rx) Stock bottle label
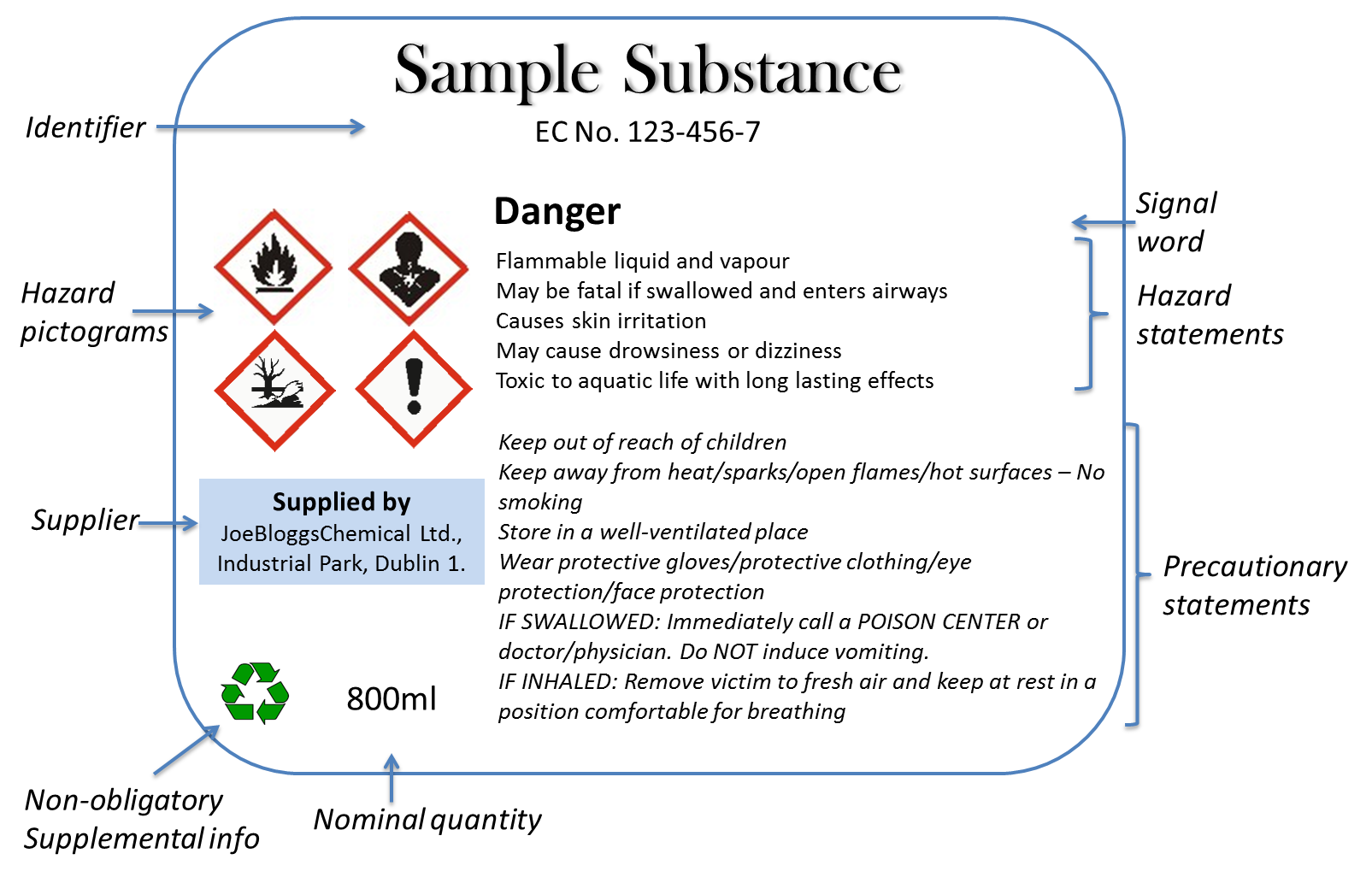
PDF WHMIS LABELS What is a WHMIS Label? - University of Regina 1. Supplier label 2. Workplace label 3. Identifier . 1. Supplier Label . This label is provided by the supplier and is a condition of sale. The supplier is the manufacturer, processor or packager of the controlled product or may be the person who imports or sells the product. An importer of controlled products has the same duties as a Title: Section 80.73 - New York Codes, Rules and Regulations 80.73 Pharmacists; dispensing schedule II substances and certain other controlled substances. (a) A licensed, registered pharmacist, or a pharmacy intern acting in conformity with the provisions of section 6806 of the Education Law and regulations thereunder in a registered pharmacy, may, in good faith and in the course of his/her professional practice, sell and dispense to an ultimate user ... NYS Pharmacy:Laws, Rules & Regulations:Article 137 - New York State ... A drug dispensed on a written or oral prescription of a physician, dentist, podiatrist or veterinarian (except a controlled substance), shall be exempt from the requirements of this section if such drug bears a label containing the name and place of business of the dispenser, the serial number and date of the prescription, directions for use as ... Hazard symbols and hazard pictograms - Chemical classification - HSE One or more pictograms might appear on the labelling of a single chemical. GB CLP hazard pictograms Explosive (Symbol: exploding bomb) Flammable (Symbol: flame) Oxidising (Symbol: flame over...
Controlled Substance Prescribing Guidelines - Ascension - Continuing ... At the conclusion of this activity, learners will be able to: Identify risks of prescribing controlled substances including opioids for pain and stimulants Develop risk management strategies to safely prescribe controlled substances for various conditions Pharmacology Study Guide Flashcards | Quizlet Which identifier is used to mark the label of a controlled substance? "C" followed by a roman numeral Which of the following is the best way to dispose of expired medications? Take medications to the pharmacy for incineration Which classification of controlled substances contains drugs that are not approved for medical use? Schedule I DOC Department of Veterans Affairs, Controlled Substances Supervisor's User ... In Controlled Substances, the Electronic Signature functionality will be used for identification for pharmacy personnel, nursing personnel, and narcotic inspectors. Identification will be used to reconfirm the identity of the user at the keyboard before proceeding with the next step in a critical process. DCMI: DCMI Metadata Terms - Dublin Core 20-01-2020 · Label: Identifier: Definition: An unambiguous reference to the resource within a given context. Comment: Recommended practice is to identify the resource by means of a string conforming to an identification system. Examples include International Standard Book Number (ISBN), Digital Object Identifier (DOI), and Uniform Resource Name (URN).
Chapter 7 | Defense Security Cooperation Agency Identifies address to receive billing from carrier if other than from ship-to addressee upon delivery of materiel. Used only for shipments that qualify for collect delivery. 9. Other. Identifies deleted MAPAD and cross-references to the MAPAD to be used in its place. M. Mark-for. Used to identify a clear text mark-for address for freight shipments.
EUR-Lex - 32017R0745 - EN - EUR-Lex - Europa Any device which, when placed on the market or put into service, incorporates, as an integral part, a substance which, if used separately, would be considered to be a medicinal product as defined in point 2 of Article 1 of Directive 2001/83/EC, including a medicinal product derived from human blood or human plasma as defined in point 10 of ...
WHMIS 1988 - Labelling Requirements : OSH Answers - Canadian Centre for ... A supplier label must: appear on all controlled products received at workplaces in Canada ; contain the following information: product identifier (name of product) supplier identifier (name of company that sold it) a statement that an MSDS is available ; hazard symbols [the pictures of the classification(s)]
DEA Numbers | Prescription Identifiers for Controlled Substances DEA numbers are assigned to healthcare professionals by the Drug Enforcement Administration, allowing these providers to prescribe controlled substances. DEA numbers follow a defined structure: 2 initial letters; Followed by 6 digits; Concluding with 1 check digit
Pharmacy Prescription Requirements - StatPearls - NCBI Bookshelf To prescribe medication, a clinician must have a DEA (Drug Enforcement Administration) license; to fill a prescription, a pharmacist must also have a controlled substance license. schedule I medications (e.g., heroin), are unable to be prescribed or filled by a pharmacist because they have no indicated medical use in the USA.
Medical Pharmacology Part Two AES Flashcards | Quizlet Which identifier is used to mark the label of a controlled substance? "C" followed by a Roman numeral With whom must a medical provider register in order to prescribe, dispense, or administer controlled substances? Drug Enforcement Agency (DEA) How often should the inventory of controlled substances take place at a medical facility? Once a day
eCFR :: 21 CFR Part 1304 -- Records and Reports of Registrants 06-04-2009 · On the effective date of a rule by the Administrator pursuant to §§ 1308.45, 1308.46, or 1308.47 of this chapter adding a substance to any schedule of controlled substances, which substance was, immediately prior to that date, not listed on any such schedule, every registrant required to keep records who possesses that substance shall take an inventory of all stocks of …
10 Types of WHMIS Labels and Their Symbol Meanings The WHMIS safety symbol is represented by an exploding bomb. It indicates that the materials pose a severe fire and explosion hazard. Since these products are highly reactive and very volatile, they are rarely used in most workplaces. Any items with this label must be managed and stored under the strictest supervision.
Drug Safety Quiz Flashcards | Quizlet Which identifier is used to mark the label of a controlled substance? "C" followed by a Roman numeral What is the best way to dispose of an expired controlled substance? By using a DEA-approved disposal company Which of the following is the best way to dispose of expired medications? Give expired medications to patients.
Investigational Medicinal Product (IMPD) Guideline - Pharma … 31-10-2020 · Applicable in case the product to be used in clinical trial purposes. An approved label with the above contents shall be received from the respective department of FDD. The format of the label shall be as mentioned in Annexure –2, 3 & 4. In case expiry dates are to be changed, an additional label shall be affixed indicating the expiry date.
Guidance on Drug Establishment Licences (GUI-0002) 01-04-2020 · Label Any legend, word or mark attached to, included in, belonging to or accompanying any food, drug, cosmetic, device or package. (Section 2 of the FDA). The action of labelling refers to affixing the inner or outer label to the drug. (FDR C.01A.001) Licensable activity Activities that require a licence (DEL).
Investigational Medicinal Product (IMPD) Guideline - Pharma ... Oct 31, 2020 · Applicable in case the product to be used in clinical trial purposes. An approved label with the above contents shall be received from the respective department of FDD. The format of the label shall be as mentioned in Annexure –2, 3 & 4. In case expiry dates are to be changed, an additional label shall be affixed indicating the expiry date.
Dy-Mark Spray & Mark Water Based All Colours Dy-Mark Dy-Mark Chemwatch Hazard Alert Code: 4 Dy-Mark Spray & Mark Water Based All Colours Chemwatch: 04-0171 Version No: 14.1.1.1 Safety Data Sheet according to WHS and ADG requirements Issue Date: 31/08/2020 Print Date: 05/11/2020 S.GHS.AUS.EN SECTION 1 Identification of the substance / mixture and of the company / undertaking Product Identifier
PDF HANDLING AND PACKAGING CONTROLLED SUBSTANCES - St. Petersburg Police ... 4. Immediately after determining the amount of controlled substances, the items should be properly marked and packaged. a. The seizing Officer should mark the evidence with: 1) The Officer's initials and payroll number; 2) Date; 3) Offense number. b.
PDF CONTROLLED SUBSTANCES (CS) - Veterans Affairs Controlled Substance Balances Report indicates a menu option. • Screen prompts will be denoted with quotation marks around them. Example: "Select INPATIENT SITE NAME" indicates a screen prompt. • Responses in bold face indicate what the user is to type in. Example: Okay to Continue? No// Y ES.
NYS Pharmacy:Laws, Rules & Regulations:Article 137 - New York … 04-03-2022 · A drug dispensed on a written or oral prescription of a physician, dentist, podiatrist or veterinarian (except a controlled substance), shall be exempt from the requirements of this section if such drug bears a label containing the name and place of business of the dispenser, the serial number and date of the prescription, directions for use as may be stated in the …
PDF DOT CHART 16 Hazardous Materials Markings,Labeling and Placarding Guide Hazardous Materials Warning Labels Actual label size: at least 100 mm (3.9 inches) on all sides CLASS 3 Flammable Liquid CLASS 4 Flammable Solid, Spontaneously Combustible, and Dangerous When Wet: Divisions 4.1, 4.2, 4.3 Cargo Aircraft Only §172.405(b), §172.415, §172.416, §172.417 CLASS 5 Oxidizer, Organic Peroxide: Divisions 5.1 and 5.2
EUR-Lex - 32017R0745 - EN - EUR-Lex - Europa Any device which, when placed on the market or put into service, incorporates, as an integral part, a substance which, if used separately, would be considered to be a medicinal product as defined in point 2 of Article 1 of Directive 2001/83/EC, including a medicinal product derived from human blood or human plasma as defined in point 10 of Article 1 of that Directive, and that has an …
Identification, Classification and Labelling of Chemicals ... not mandated. The UN RTDG label defines hazards by the use of symbols, colours and danger warning words for specific hazards (explosive, radioactive, corrosive, etc.). 1. Chemical identity 2. Hazard symbol and indication of danger 3. Risk phrases 4. Safety phrases 5. Supplier identification (full address and tel. No.) 6. EEC number if allocated
Dy-Mark Spray & Mark Water Based All Colours Dy-Mark Dy-Mark Chemwatch Hazard Alert Code: 4 Dy-Mark Spray & Mark Water Based All Colours Chemwatch: 04-0171 Version No: 14.1.1.1 Safety Data Sheet according to WHS and ADG requirements Issue Date: 31/08/2020 Print Date: 05/11/2020 S.GHS.AUS.EN SECTION 1 Identification of the substance / mixture and of the company / undertaking Product Identifier
Maritime Occupational Health and Safety Regulations 4 (1) If an employer is required by section 125 or 125.1 of the Act to the employer must keep and maintain the record and make it readily available for examination by the Head of Compliance and Enforcement, the policy committee or, if there is no policy committee, the work place committee or the health and safety representative for the vessel to which it applies.
EUR-Lex - 32008R1272 - EN - EUR-Lex - Europa If a substance is subject to harmonised classification and labelling in accordance with Title V through an entry in Part 3 of Annex VI, that substance shall be classified in accordance with that entry, and a classification of that substance in accordance with Title II shall not be performed for the hazard classes or differentiations covered by that entry.
Drug Identification, Kentucky State Police Evidence Manual on ... Evidence Collection Handbook - DRUG IDENTIFICATION. Drug evidence includes powders, liquids, tablets, capsules, or plan samples suspected of being or containing legally controlled substances. It includes marijuana, peyote, opium, LSD, heroin, and cocaine. This evidence does not include the identification of drugs in blood, urine, or pathological specimens; these samples are categorized under Toxicology (see the Toxicology Section).
Guidance on Drug Establishment Licences (GUI-0002) Apr 01, 2020 · Controlled drug A substance included in Schedule I, II, III, IV or V of the Controlled Drugs and Substances Act. Distributor. A person, including an association or partnership, who under their own name, or under a trade, design or word mark, trade name or other name, word or mark controlled by them, sells a food or drug.
Implementation Guidelines for Alcohol and Drug Regulations - Chapter 6 Identification of either a controlled substance or its metabolite in the urine indicates use of the controlled substance in the recent past. A metabolite is a modified form of a controlled substance that has been chemically altered by the body's metabolic system. ... and properly label and preserve the chain of custody of specimens.
WHMIS 2015 - Labels : OSH Answers - Canadian Centre for Occupational ... Product identifier - the brand name, chemical name, common name, generic name or trade name of the hazardous product. Initial supplier identifier - the name, address and telephone number of either the Canadian manufacturer or the Canadian importer*. Pictogram (s) - hazard symbol within a red "square set on one of its points".
Medical Assisting Pharmacology Test Flashcards | Quizlet Which identifier is used to mark the label of a controlled substance? "C" followed by a Roman numeral How often should the inventory of controlled substances take place at a medical facility? Once a day What is the best way to dispose of an expired controlled substance? By using a DEA-approved disposal company What does it mean to dispense a drug?
PDF Controlled Substance FAQ - CVMA InLine Controlled substances are clearly marked with a "C" on the vial, syringe, or container that they are packaged in by the manufacturer. If you are unsure as to whether or not a substance in controlled, you can also check the Federal Controlled Substances Act (CSA) drug schedules.
CFR - Code of Federal Regulations Title 21 - Food and Drug ... Mar 29, 2022 · (i) For each controlled substance in bulk form to be used in (or capable of use in) the manufacture of the same or other controlled or non-controlled substances in finished form, the inventory shall include: (A) The name of the substance and (B) The total quantity of the substance to the nearest metric unit weight consistent with unit size.














/200415150-001-56a6fd333df78cf772914d1a.jpg)






Post a Comment for "43 which identifier is used to mark the label of a controlled substance"