44 hybridization of so2
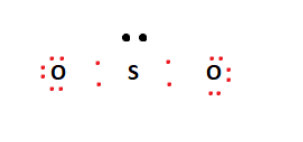
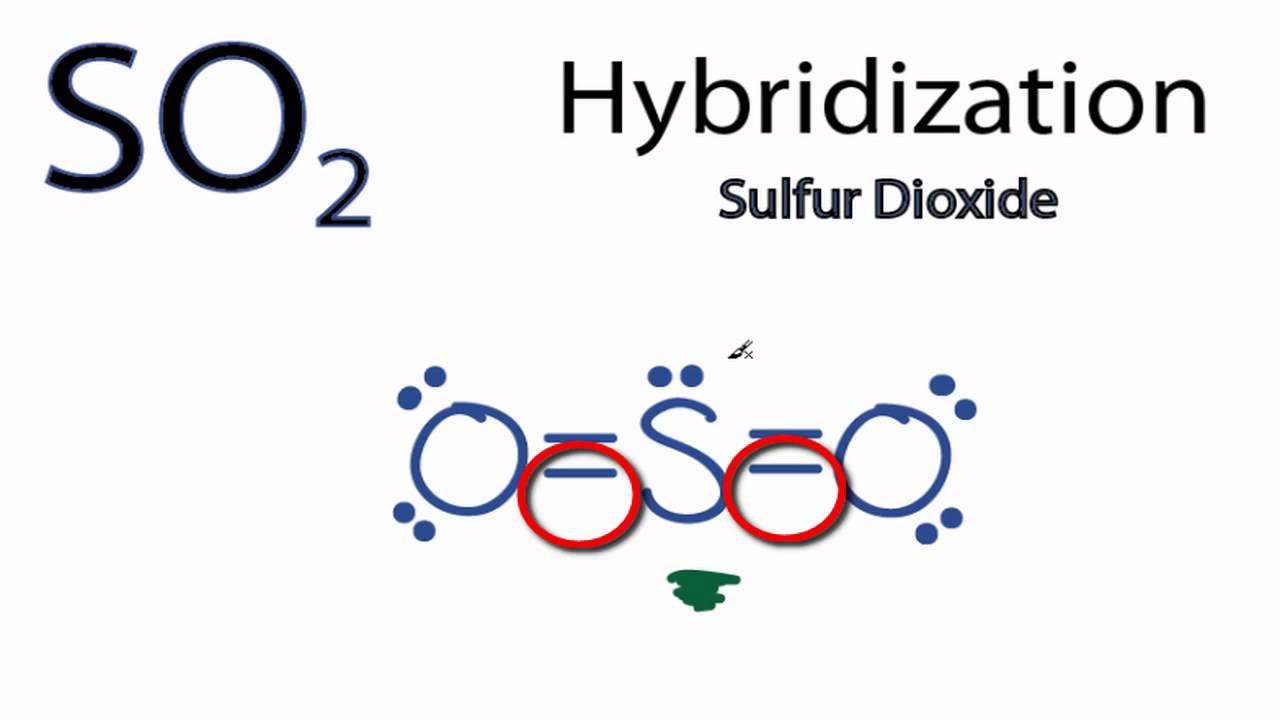
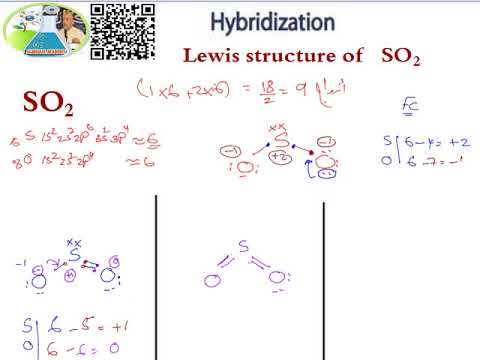
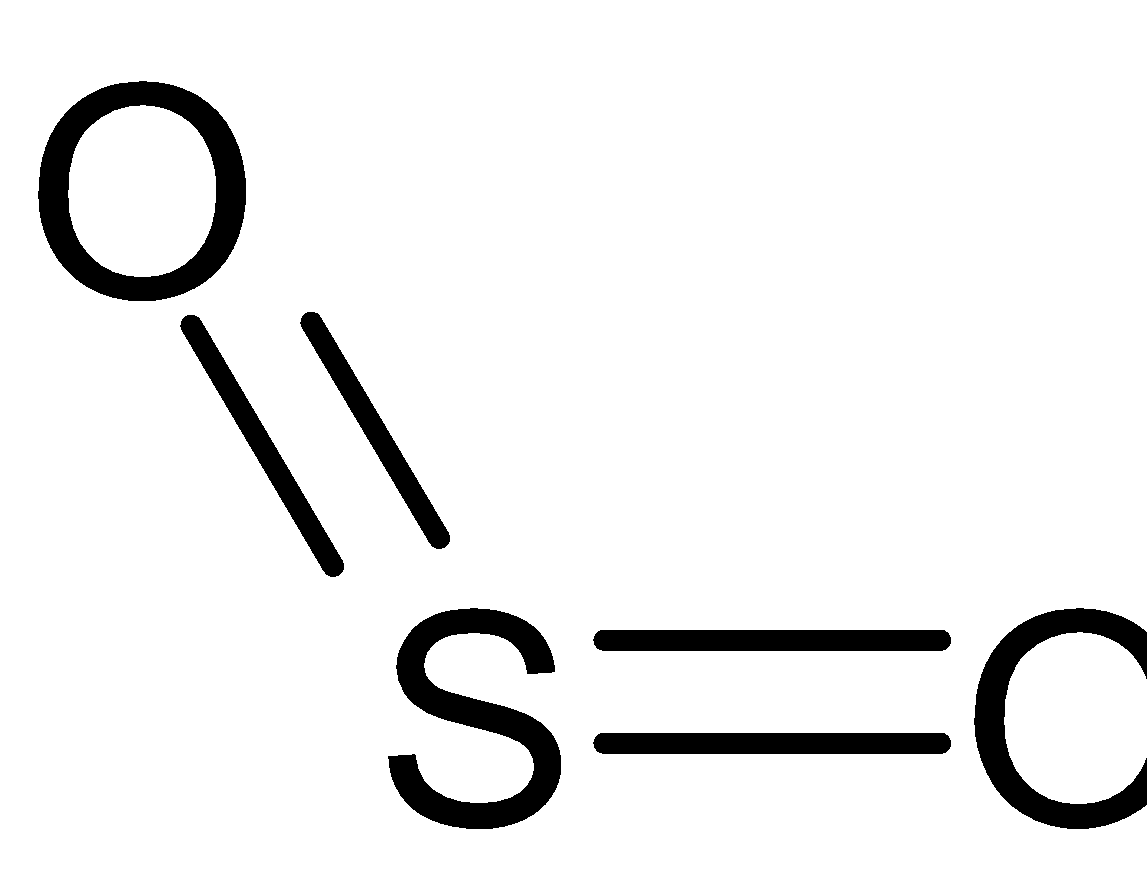
inorganic chemistry - Hybridization of sulfur in sulfur dioxide ... The structure of sulfur dioxide ($\ce{SO2}$) is quite complicated.The image from socratic.org * explains it quite well, please find the $\ce{SO2}$ hybridization diagram via the Internet Archive.. As seen, all the atoms have $\ce{sp^2}$ hybridization.. I'll only focus on the central sulfur atom. Two $\ce{sp^2}$ orbitals form $\ce{\sigma}$-bonds with the two oxygens. SO2 Lewis Structure: Drawings,Hybridization,Shape,Charges,Pair And ... SO2 hybridization: The hybridization takes place in SO2 is sp2 type . For formation of SO2 ,we need 2 double bond outside the sulfur atom, while sulfur is in the middle or central. During formation of SO2 , the hybridization used is SP2. There are 2 sigma, 2 pi and one lone pair of electrons. The SO2 is in bent shape.
So2 hybridization SO2 hybridization: The hybridization takes place in SO2 is sp2 type . For formation of SO2,we need 2 double bond outside the sulfur atom, while sulfur is in the middle or central. During formation of SO2, the hybridization used is SP2. There are 2 sigma, 2 pi and one lone pair of electrons. The SO2 is in bent shape.
Hybridization of so2
Answered: 5. What is the hybridization of the… | bartleby Science Chemistry Q&A Library 5. What is the hybridization of the oxygen atoms in SO2? airs of electrons are in the o-framework of a. Sulfur dioxide, SO2, has a total of 9 pairs of electrons. So2 hybridization Search: Hybridization Of H2s. In NH3 - There is 1 lone pair and hence due to Lp Bp repulsion the angle decreases a little Genetic evidence for hybridization between the native Spartina maritima and the introduced Spartina alterniflora (Poaceae) in South-West France: Spartina × Pershina L sp hybridization : sum of attached atoms + lone pairs ... Hybridisation in SO2 molecule is: - toppr.com >> Hybridization >> Hybridisation in SO2 molecule is: ... Verified by Toppr. Correct option is B) In Sulphur Dioxide, the S atom has 2 bond pairs and 1 lone pair, so it requires 3 hybrid orbitals. So the hybridisation is s p 2. Solve any question of Chemical Bonding and Molecular Structure with:-
Hybridization of so2. So2 hybridization The hybridization of sulphur in SO2 is - 52687672 ashwink2501 ashwink2501 17 hours ago CBSE BOARD XII Secondary School The hybridization of sulphur in SO2 is A sp B sp3 C sp2 D dsp2 ashwink2501 is waiting for your help. Add your answer and. colorado optometry license requirements SO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram H = 3 = Sp2 hybridization. I hope the hybridization of SO2 is clear from both the explained concepts. SO2 Molecular geometry The molecular geometry of SO2 is bent, with a bond angle of 120°. We can easily find out the molecular geometry of any compound using the given chart. Here, A = central atom, X = surrounding atoms and E = the lone pairs. How is the hybridization of SO3 2-determined? - Quora The hybridization of O2, N2, and F2 are sp2, sp, sp3 respectively. O2 So, on the basis of this we can say that O2 has sp2 hybridization.. N2 We already know that N-N has triple bond in its stable condition.There are one sigma bond and two pi bonds. So, we can say that the N2 has sp hybridization. F2 Hybridization of NO2 - Introduction, Lewis Structure, Molecular ... Nitriteornitroion is the sp2 hybridized as well, and here, the lone pair orbital is hybridized. It is to note that both the N-O bonds are equivalent because of the resonance. Even the double bonds behave similarly to lone pairs for the repulsions, in effect (also, note that SO2 has a bond angle of approx 120°). Here, the bond angle in NO2- is 115o.
Hybridization of sulfur | Physics Forums 468. 1. Hybridization of carbon. Pretty sure my professor is wrong once again. SO2 should have two double bonds which gives the sulfur a minimal formal charge and two signs bonds. The two sigma bonds imply sp hybridization, not the blatantly wrong circled answer. Last edited: Oct 18, 2013. so2 hybridization explained - Get Education The hybridization of SO2 is Sp2. Now can determine the hybridization of SO2 in 2 ways, one is the theory, and the second is the straight applying the formula. I would undoubtedly suggest recognizing the concept first and afterwards. You can opt for the procedure. SO4 2- Lewis Structure, Hybridization, Bond Angle and Molecular Geometry Hence SO42- ion has an sp3 hybridization. SO42- Molecular Geometry We can determine the molecular geometry of any given molecule using the VSEPR theory model and the AXN notation method. For example, for the Sulphate ion, the AXN notation would be AX4, as it forms bonds with four oxygen atoms. SiO2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity Conclusion. SiO2 has a net dipole moment of zero. It has a linear electron and molecular geometry with a bond angle of 180 degrees and a hybridization of Sp. The Silicon dioxide Lewis structure has a total of 16 valence electrons. In the Lewis dot structure of SiO2, the formal charge is zero.
SO2 Hybridization (Sulfur Dioxide) - YouTube Hello Everyone!Today in this video we are going to help you find out the hybridization of SO2 molecule. It is a chemical formula for Sulfur Dioxide. To find ... SO2 Hybridization - YouTube A description of the hybridization of SO2 including sigma and pi bonds.Note that the SO2 hybridization is sp2 for the central sulfur atom. It's also sp2 fo... The hybridization of S in SO2 is: - toppr.com class 12. Atoms Chemical Kinetics Moving Charges and MagnetismMicrobes in Human Welfare Semiconductor Electronics: Materials, Devices and Simple Circuits. SO2 Molecular Geometry, Hybridization, Lewis Structure & MO Diagram SO2 Hybridization Now coming to the formula part. The formula for finding hybridization of any compound is; H = ½ [ V+M-C+A] Where, H depicts Hybridization V is the no. of valence electrons M is the count of monovalent atoms present C represents the cationic charge A represents the anionic charge Here, if H is 2, it's Sp hybridization.
What is the hybridization in "CO"_2? | Socratic Explanation: You must first draw the Lewis structure for CO2. According to VSEPR theory, we can use the steric number (SN) to determine the hybridization of an atom. SN = number of lone pairs + number of atoms directly attached to the atom. SN = 2 corresponds to sp hybridization. SN = 3 corresponds to sp2 hybridization.
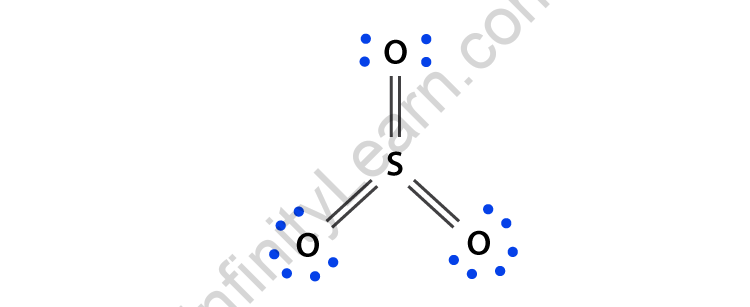
Hybridization of SO2 - Hybridization of S in Sulphur Dioxide In SO 2 hybridization two 3p orbitals and one 3s orbital get hybridized. Two-hybrid orbitals contain unpaired electrons and one hybrid orbital will have the lone pair. The 3d and 3p orbitals remain the same, and they form pi bonds. SO 2 Molecular Geometry And Bond Angles SO 2 molecular geometry is considered to V-shaped or bent.
Hybridization of SO₂ - Definition, Electron Geometry Vs ... - VEDANTU The hybridization of the two oxygen atoms is sp2 as properly. SO2 is a bent shape (molecular geometry). Sulfur desires 6 electrons, and so does oxygen. Consequently, 6×three=18 valence electrons distribute in the course of the structure placing 4 for every of two double bonds makes use of up to eight.
SO2(Sulfur Dioxide) Lewis Structure, Hybridization, Molecular Geometry ... SO2 comprises a Sulfur atom surrounded by two oxygen atoms. In its most stable state, the Sulfur atom forms double bonds with the adjacent oxygen atoms. There is also a lone pair above the Sulfur atom. The hybridization of the SO2 is given by sp2. SO2 has a Bent molecular structure and shape with bond angles of 120°. About Priyanka
Hybridization of SO3 (Sulphur Trioxide): Hybridization of S in SO3 To understand the hybridization of sulphur trioxide we have to understand the bonding between sulphur and oxygen. If we draw and look at the Lewis structure sulphur will be the central atom and will have three double bonds with oxygen. In this, there is one sigma and one pi bond formed. This gives us the sp 2 hybridization type.
SO2 Lewis Structure, Molecular Geometry, Hybridization, Polar or ... SO2 has an SP2 type hybridization. We can easily determine any of the molecule molecular orbital (i.e., hybridization) with the help of given formula which is, No. of hybridization = [V + X - C + A]/2 where, V = No. of valence electrons of central atom X = No. of monovalent atoms around the central atom C = Positive charge on cation
What Is SO2 Hybridization? - Reference.com SO2, commonly known as sulfur dioxide, has an sp3 hybridization. The molecular geometry of sulfur dioxide consists of two oxygen atoms bonded to the central sulfur atom. Hybridization explains the molecular structure of a compound.
Hybridisation in SO2 molecule is: - toppr.com >> Hybridization >> Hybridisation in SO2 molecule is: ... Verified by Toppr. Correct option is B) In Sulphur Dioxide, the S atom has 2 bond pairs and 1 lone pair, so it requires 3 hybrid orbitals. So the hybridisation is s p 2. Solve any question of Chemical Bonding and Molecular Structure with:-
So2 hybridization Search: Hybridization Of H2s. In NH3 - There is 1 lone pair and hence due to Lp Bp repulsion the angle decreases a little Genetic evidence for hybridization between the native Spartina maritima and the introduced Spartina alterniflora (Poaceae) in South-West France: Spartina × Pershina L sp hybridization : sum of attached atoms + lone pairs ...
Answered: 5. What is the hybridization of the… | bartleby Science Chemistry Q&A Library 5. What is the hybridization of the oxygen atoms in SO2? airs of electrons are in the o-framework of a. Sulfur dioxide, SO2, has a total of 9 pairs of electrons.
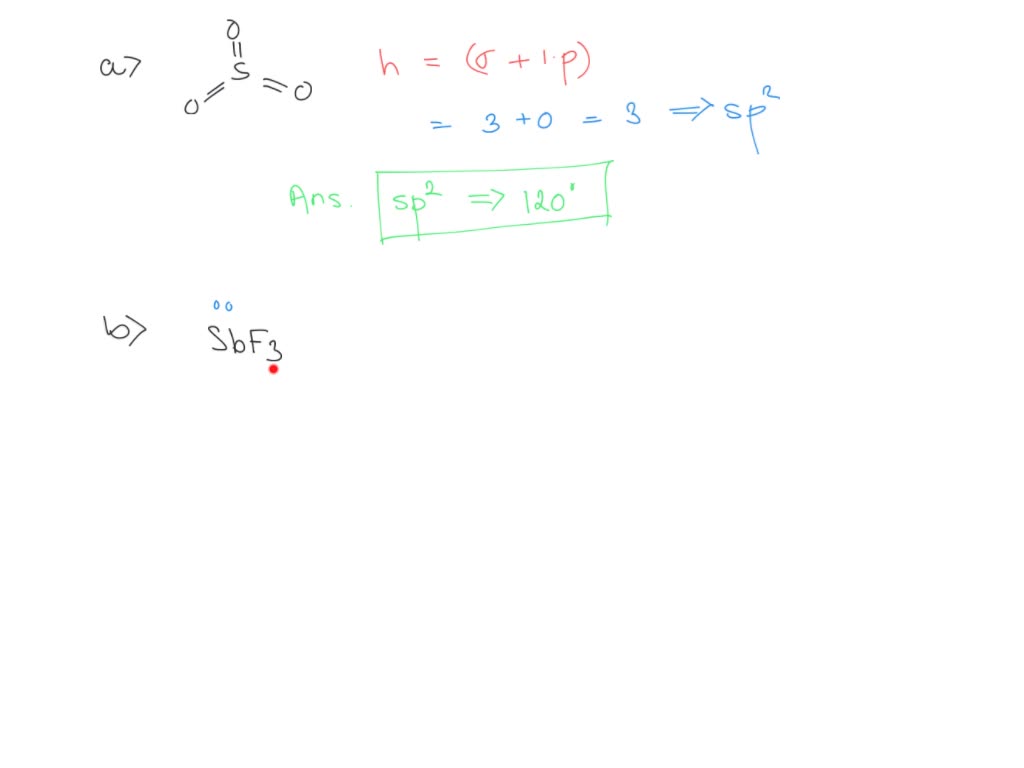
what is the hybridization of the central atom in so2 hybridization what are the approximate bond angles in this substance bond angles what is the hybridization of the central atom in sbf3 hy 02773






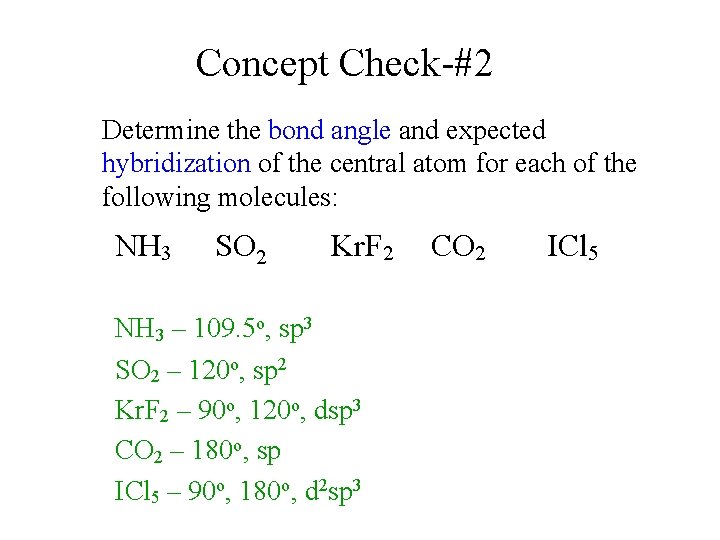




















Post a Comment for "44 hybridization of so2"